What are Dynochem Excel custom functions?
Dynochem Excel custom functions retrieve properties data from the materials system or perform calculations directly in worksheet cells and are available when Excel is open. The following functions are available (those new in Dynochem 6 are in bold):
- DC_GetProperty
- DC_GetTDepProperty
- DC_MixtureBinary
- DC_MixtureTernary
- DC_ActivityBinary
- DC_ActivityTernary
- DC_SourceBIPs
- DC_GetH2Henry
- DC_CalcMW
- DC_CalcMIMass
- DC_Format
- DC_Visvaligam
- DC_Regression
- DC_Prediction
- DC_ConvertUnit

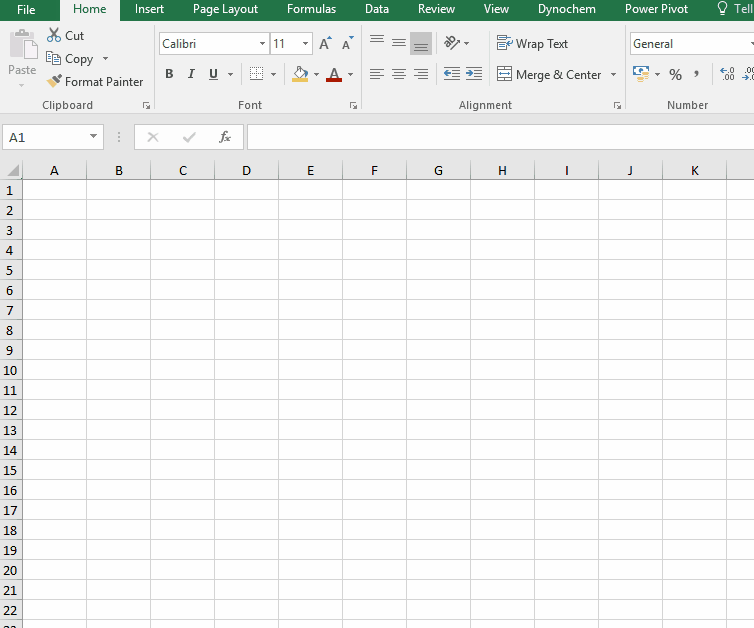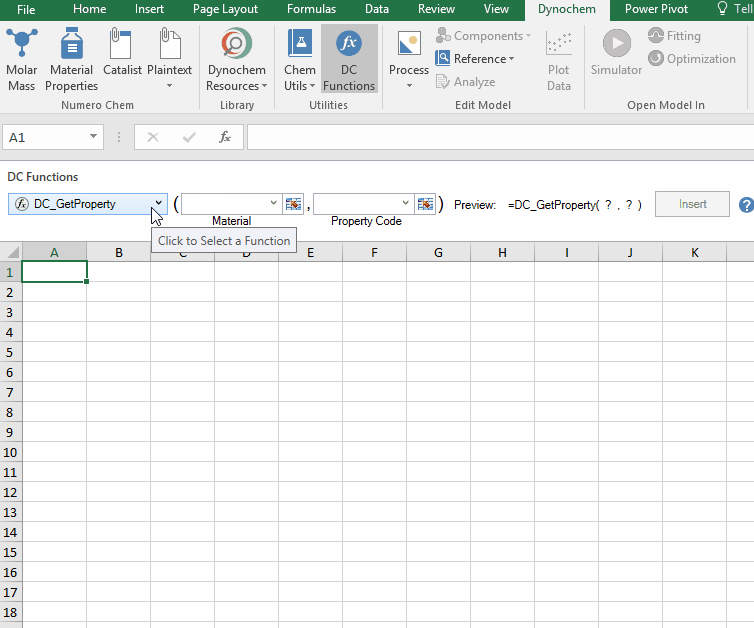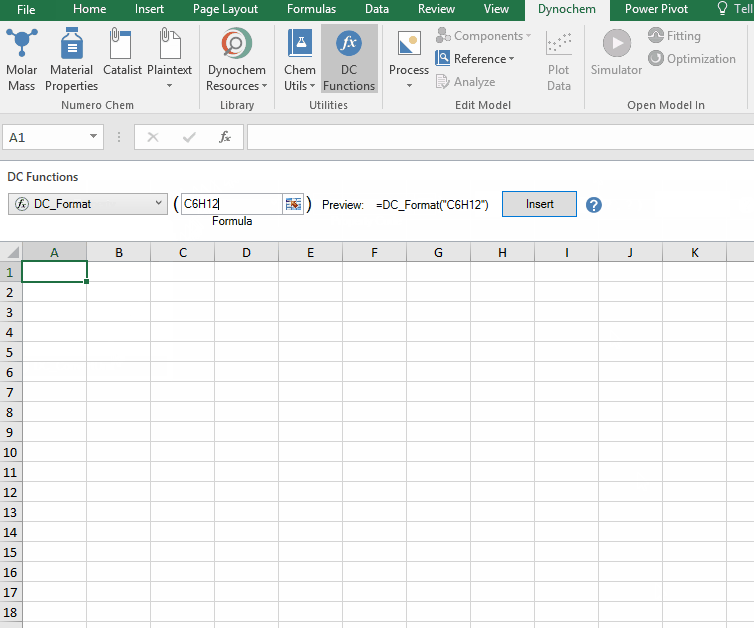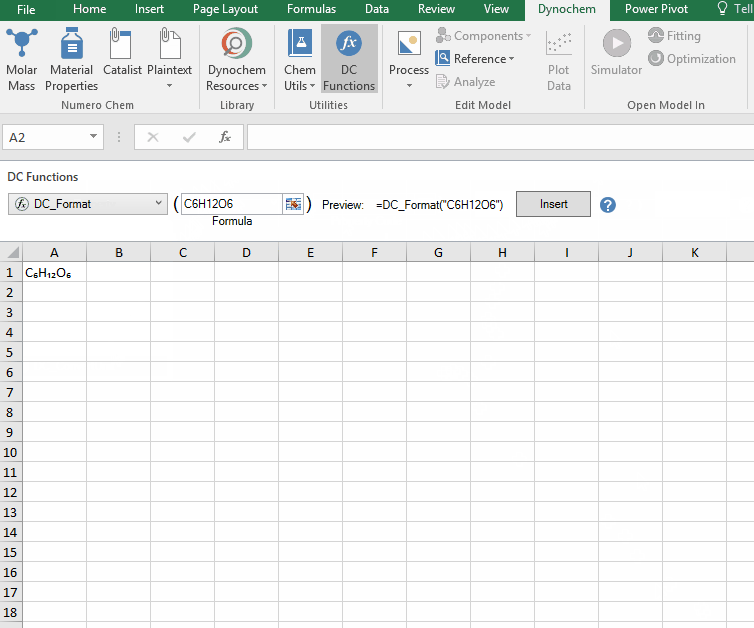
DC_GetProperty function
The DC_GetProperty function allows you to search for temperature independent properties (static properties) such as a solvent's critical temperature, triple point temperature or normal boiling point.
Syntax
• DC_ GetProperty(“material”, “property code”)
| Argument name |
Description |
| Material (required) |
The material whose property that you want |
| Property (required) |
The property that you want |
Example:
Finding the melting point for EtOH, EtOAc, DMF and DMSO.
List of Property codes
| Property code |
Property |
Property code |
Property |
| Antoines |
Antoines |
FLVL |
Lower Flammability Limit % |
| UNIFAC VLE |
Text showing UNIFAC VLE fragmentation |
FLTL |
Lower Flammability Limit Temperature |
| UNIFAC LLE |
Text showing UNIFAC LLE fragmentation |
FLVU |
Upper Flammability Limit % |
| UNIFAC Modified |
Text showing UNIFAC modified fragmentation |
FLTU |
Upper Flammability Limit Temperature |
| CAS |
CAS Registry Number |
AIT |
Auto Ignition Temperature |
| MW |
Molar mass |
HSUB |
Heat of Sublimation |
| MI_MASS |
Monoisotopic mass |
ACEN |
Acentric Factor |
| LINEAR_FORMULA |
Linear formula |
RG |
Radius of Gyration |
| FORMATTED_LINEAR_FORMULA |
Formatted linear formula |
SOLP |
Solubility Parameter |
| HILL_FORMULA |
Hill formula |
DM |
Diplole Moment |
| FORMATTER_HILL_FORMULA |
Formatted Hill formula |
VDWV |
van der Waals Reduced Volume |
| SMILES |
SMILES representation |
VDWA |
van der Waals Reduced Area |
| InChI |
IUPAC international chemical identifier |
RI |
Refracrtive Index |
| InChIKEY |
Hashed version of the full standard InChI |
PAR |
Parachor |
| FAMILY |
Solvent family |
DC |
Dielectric constant |
| RVALUE |
Relative Van der Waals volume, R |
Hilde |
Hildebrand solubility parameter |
| QVALUE |
Relative Van der Waals surface area, Q |
HAND |
Hansen dispersion forces |
| MP |
Melting Point at 1atm |
HANP |
Hansen polar forces |
| NBP |
Normal Boiling Point |
HANH |
Hansen hydrogen bonds |
| TPT |
Triple Point Temperature |
ICH_CLASS |
ICH Residual Solvent Class |
| TPP |
Triple Point Pressure |
SOLVENT_TYPE |
Solvent Type including ACS Class |
| TC |
Critical Temperature |
ACS_SAFETY |
ACS Greenness Safety |
| PC |
Critical Pressure |
ACS_HEALTH |
ACS Greenness Health |
| VC |
Critical Volume |
ACS_ENV_AIR |
ACS Greenness Env (Air) |
| ZC |
Critical Compressibility Factor |
ACS_ENV_WATER |
ACS Greenness Env (Water) |
| LVOL |
Liquid Molar Volume at 298.15 K |
ACS_ENV_WASTE |
ACS Greenness Env (Waste) |
| HFOR |
Enthalpy of Formation for Ideal Gas |
GSK_CLASS |
GSK Guide 2009 Solvent Class |
| GFOR |
Gibbs Energy of Formation for Ideal Gas |
GSK_FLAMM |
GSK Guide 2009 Flammability & Explosion |
| ENT |
Absolute Entropy of Ideal Gas |
GSK_REACT |
GSK Guide 2009 Reactivity/Stability |
| HSTD |
Standard State Enthalpy of Formation |
GSK_HEALTH |
GSK Guide 2009 Health |
| GSTD |
Standard State Gibbs Energy of Formation |
GSK_WASTE |
GSK Guide 2009 Waste |
| SSTD |
Standard State Absolute Entropy |
GSK_ENV_IMP |
GSK Guide 2009 Environmental impact |
| HFUS |
Enthalpy of Fusion at Melting point |
GSK_LIFECYC |
GSK Guide 2009 Life Cycle Score |
| HCOM |
Net Standard Enthalpy of Combustion |
GSK_LEGAL_FLAG |
GSK Guide 2009 Legislation Flag |
| FP |
Flash Point |
GSK_EHS_FLAG |
GSK Guide 2009 EHS Red Flag |
For further details about the ACS solvent selection guide, visit the following ACS link. The guide assigns a score from 1 to 10 for each solvent under the respective categories, with a score of 10 being of most concern and a score of 1 suggesting few issues. For the ACS scores, 1=good and 10=bad.
For further details about the GSK solvent selection guide, visit the following link. Each solvent is scored from 1 (red) to 10 (green) to give a relative ranking for every solvent in the guide in each category, where the score is based on data or a physical observable property. Supplementary information is available here. In the GSK scores, 1=bad and 10=good.
DC_GetTDepProperty function
The DC_GetTDepProperty allows you to search for temperature dependent properties for materials such as liquid density and liquid viscosity. In its simplest form it says:
- DC_GetTDepProperty("material name", "property code", temperature at which you want this property)
The resulting output for a DC_GetTDepProperty is the numerical value for that solvent property at that temperature.
Syntax
• DC_GetTDepProperty(“material”, “property code”, temperature
| Argument name |
Description |
| Material (required) |
The material whose property you want |
| Property (required) |
The material that you want |
| Temperature (required) |
The temperature for the property |
Example
Find the liquid density of EtOH at 25 C up to 50 C, in increments of 5 C.
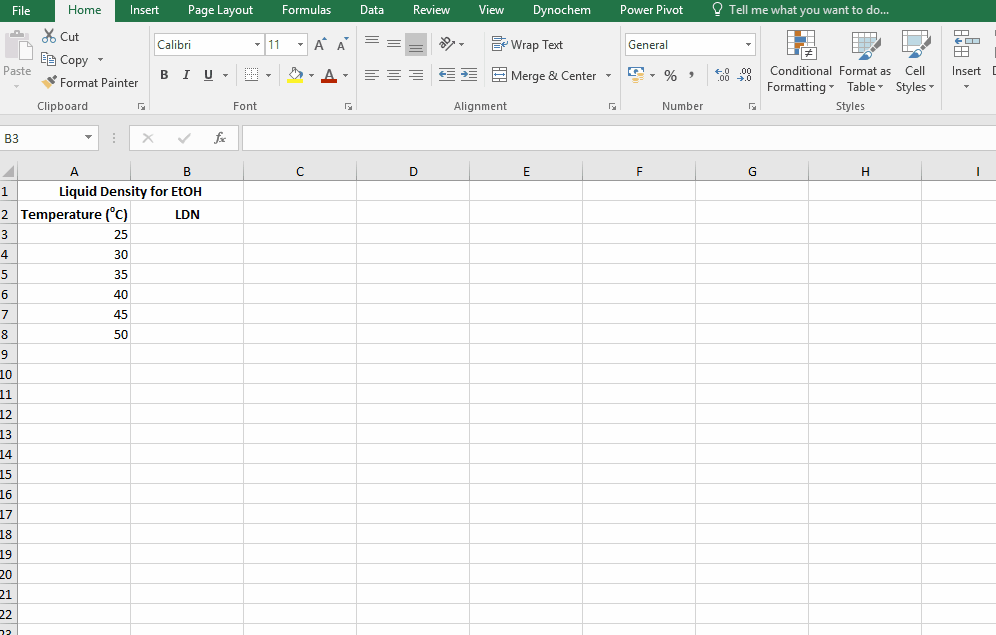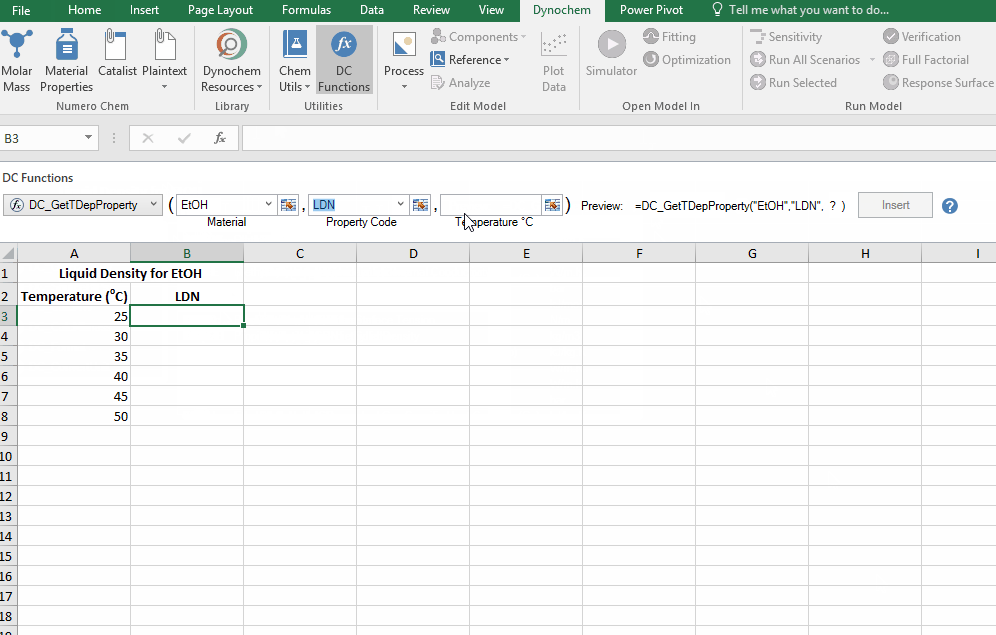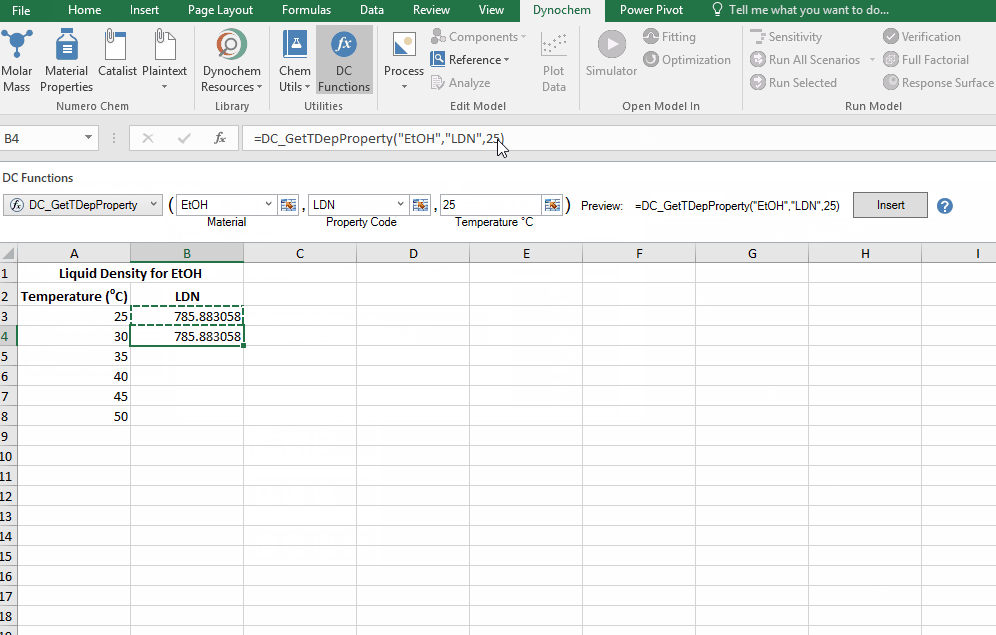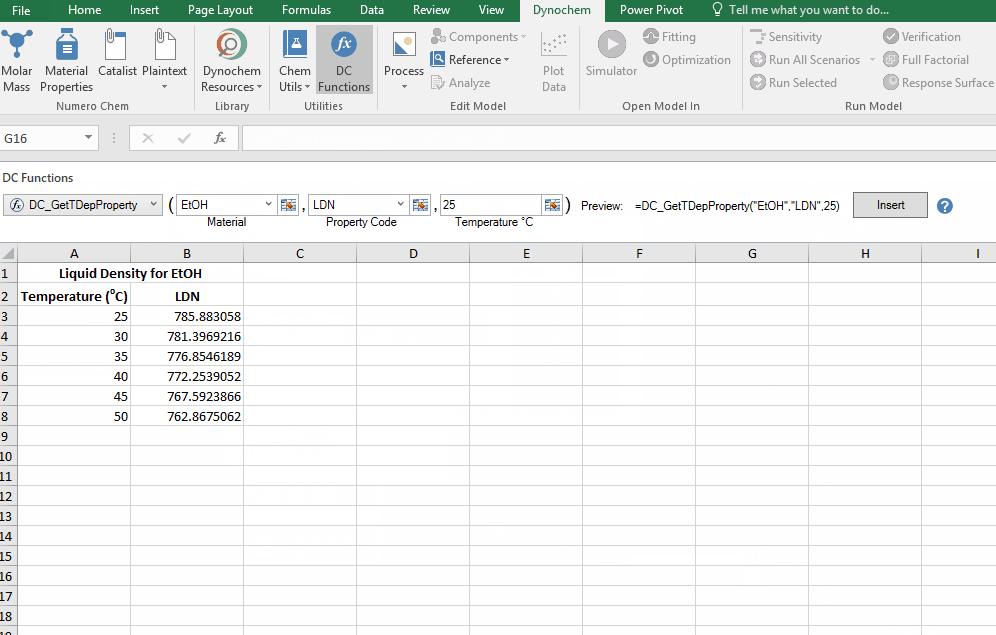
List of Property codes
| Solvent Property Code |
Solvent Property |
Units |
| LDN |
Liquid Density |
kg/m3 |
| LCP |
Liquid Specific Heat Capacity |
kJ/kg.K |
| LVS |
Liquid Viscosity |
mPa.s |
| LTC |
Liquid Thermal Conductivity |
W/m.K |
| LVP |
Vapor Pressure of the Liquid |
bar |
| HVP |
Heat of Vaporization |
kJ/mol |
| SDN |
Solid Density |
kg/m3 |
| SCP |
Solid Specific Heat Capacity |
kJ/kg.K |
| STC |
Solid Thermal Conductivity |
W/m.K |
| VTC |
Vapor Thermal Conductivity |
W/m.K |
| SVP |
Vapor Pressure of Solid |
bar |
| ICP |
Ideal Gas Specific Heat Capacity |
kJ/kg.K |
| SVR |
Second Virial Coefficient |
m3/kmol |
| VVS |
Vapor Viscosity |
mPa.s |
| SUT |
Surface Tension |
N/m |
DC_MixtureBinary function
The DC_MixtureBinary function allows you to calculate temperature-dependent properties for binary solvent mixtures such as the liquid density or heat capacity. Thermodynamic ideality is assumed when calculating these properties and components are assumed to be miscible.
Syntax
• DC_MixtureBinary("Property Code", "Temperature" , "Solvent1" , "wt% 1" , "Solvent2" , "wt% 2")
| Argument |
Description |
| Property Code |
The property you want |
| Temperature |
Real value. This is the temperature in degree Celsius |
| Solvent 1 |
Solvent name (e.g. MeOH, EtOAc, Water) |
| wt% 1 |
Real value. This is the weight % of the first solvent |
| Solvent 2 |
Solvent name |
| wt% 2 |
Real value. This is the weight % of the second solvent |
DC_MixtureTernary function
The DC_MixtureTernary function allows you to search for temperature dependent properties for ternary solvent mixtures such as the liquid density or heat capacity. Thermodynamic ideality is assumed when calculating these properties and components are assumed to be miscible.
Syntax
• DC_MixtureTernary("Property Code", "Temperature" , "Solvent1" , "wt% 1" , "Solvent2" , "wt% 2" , "Solvent3" , "wt% 3")
| Argument |
Description |
| Property Code |
The property you want |
| Temperature |
Real value. This is the temperature in degrees Celsius |
| Solvent 1 |
Solvent name (e.g. MeOH, EtOAc, Water) |
| wt% 1 |
Real value. This is the weight % of the first solvent |
| Solvent 2 |
Solvent name |
| wt% 2 |
Real value. This is the weight % of the second solvent |
| Solvent 3 |
Solvent name |
| wt% 3 |
Real value. This is the weight % of the third solvent |
DC_ActivityBinary
The DC_ActivityBinary function calculates the activity coefficients for binary solvent mixtures. Activity coefficients may be used to calculate behaviour in non-ideal systems.
Syntax
• DC_ActivityBinary("Method", "Temperature" , "Solvent1" , "Mole Fraction 1" , "Solvent2" , "Mole Fraction 2")
| Argument |
Description |
| Method |
Method (NRTL, UNIQUAC, UNIFAC VLE, UNIFAC LLE, UNIFAC modified) |
| Temperature |
Real value. This is the temperature in degree Celsuis |
| Solvent 1 |
Solvent name (e.g. Meoh, EtOAc, Water) |
| Mole Fraction 1 |
Real value. This is the mole fraction of the first solvent |
| Solvent 2 |
Solvent name |
| Mole Fraction 2 |
Real value. This is the mole fraction of the second solvent |
DC_ActivityTernary
The DC_ActivityTernary function calculates the activity coeffiicent for ternary solvent mixtures. Activity coefficients may be used to calculate behaviour in non-ideal systems.
Syntax
• DC_ActivityTernary("Method", "Temperature" , "Solvent1" , "Mole Fraction 1" , "Solvent2" , "Mole Fraction 2", "Solvent3" , "Mole Fraction 3")
| Argument |
Description |
| Method |
Method (NRTL, UNIQUAC, UNIFAC VLE, UNIFAC LLE, UNIFAC modified) |
| Temperature |
Real value. This is the temperature in degrees Celsius |
| Solvent 1 |
Solvent name (e.g. MeOH, EtOAc, Water) |
| Mole Fraction 1 |
Real value. This is the mole fraction of the first solvent |
| Solvent 2 |
Solvent name (e.g. MeOH, EtOAc, Water) |
| Mole Fraction 2 |
Real value. This is the mole fraction of the second solvent |
| Solvent 3 |
Solvent name (e.g. MeOH, EtOAc, Water) |
| Mole fraction 3 |
Real value. This is the mole fraction of the third solvent |
DC_SourceBIPs
The DC_SourceBIPS function allows you to check the source of the binary interaction parameters (BIPs) used the the built-in Materials system when estimating activity coefficients.
Syntax
• DC_SourceBIPs("solvent one", "solvent two", "method")
| Argument name |
Description |
| Solvent one (required) |
Name of the first solvent |
| Solvent two (required) |
Name of the second solvent |
| Method (required) |
NRTL/UNIQUAC |
Outputs
- Regressed to data (DDB)
- Regressed to Mod UNIFAC
- Cannot find BIPs
Example:
To determine whether the following solvent pairs have BIPs in the database:
DC_GetH2Henry
The DC_GetH2Henry allows you to search for the Henry constant for Hydrogen in (mol/m3)/(mol/m3). The resulting output for a DC_GetH2Henry is the numerical value for that solvent property at that particular temperature.
The following references are used as sources to provide results from this function:
1 J. M. SHAW, A Correlation for Hydrogen Solubility in Alicyclic and Aromatic Solvents, THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING. VOLUME 65. APRIL 1987.
2 "Thi-Kim-Hoang Trinh, Jean-Charles de Hemptinne, Rafael Lugo, Nicolas Ferrando and Jean-Philippe Passarello, Hydrogen Solubility in Hydrocarbon and Oxygenated Organic Compounds, J. Chem. Eng. Data 2016, 61, 19−34"
3 "The properties of gases & liquids” by Reid, Prausnitz and Poling (Fourth Edition, page332, section 8-11).
4 U. J. JÁUREGUI-HAZA, E. J. PARDILLO-FONTDEVILA, A. M. WILHELM and H. DELMAS, SOLUBILITY OF HYDROGEN AND CARBON MONOXIDE IN WATER AND SOME ORGANIC SOLVENTS, Latin American Applied Research 34:71-74 (2004).
5 Young, C. L. "Hydrogen and Deuterium" Solubliity Data Series Volume 5/6 Pergamon Press 1981
Syntax
• DC_GetH2Henry(“solvent”, temperature)
| Argument name |
Description |
| Solvent (required) |
The solvent whose property you want |
| Temperature (required) |
The temperature for the property |
Example:
Find the Henry constant for Hydrohen in MeOH at 30 degrees.
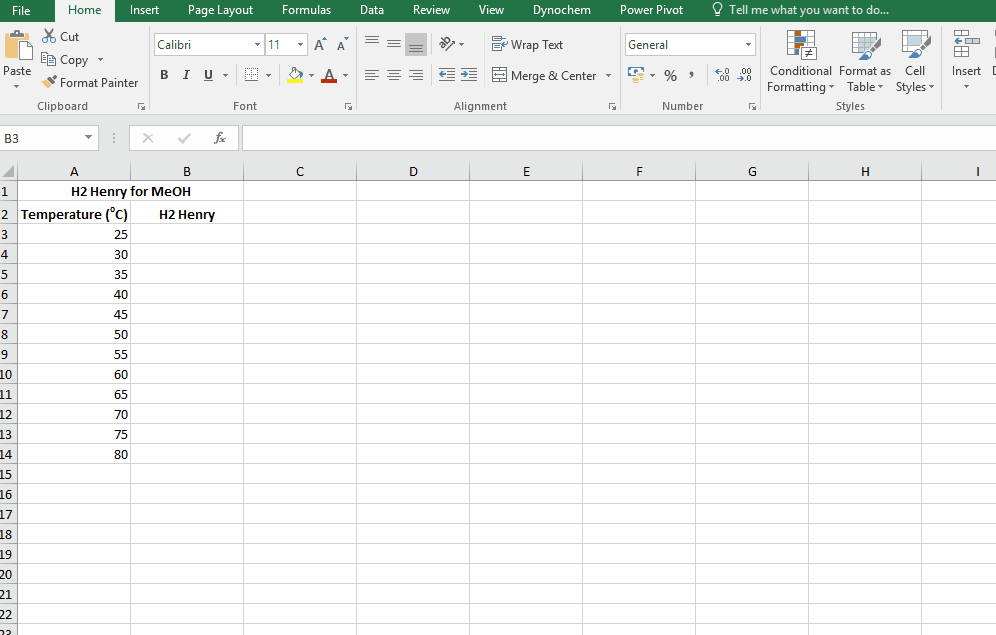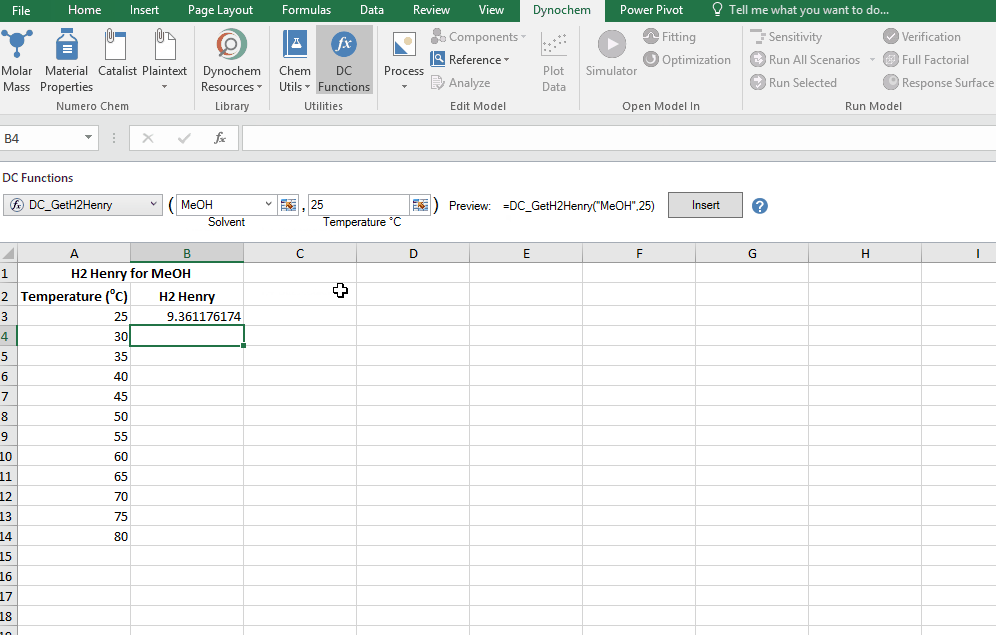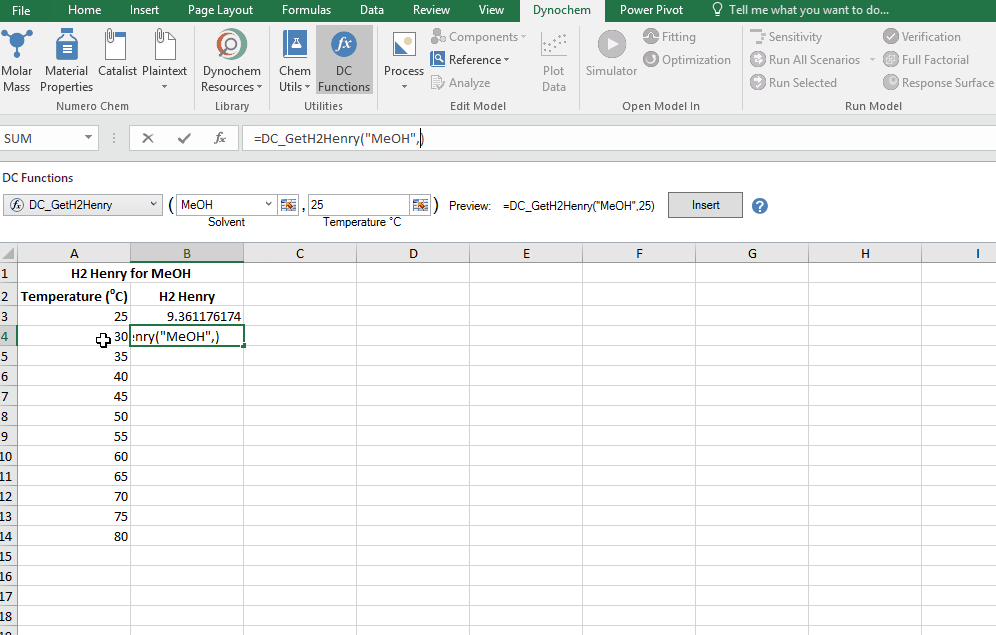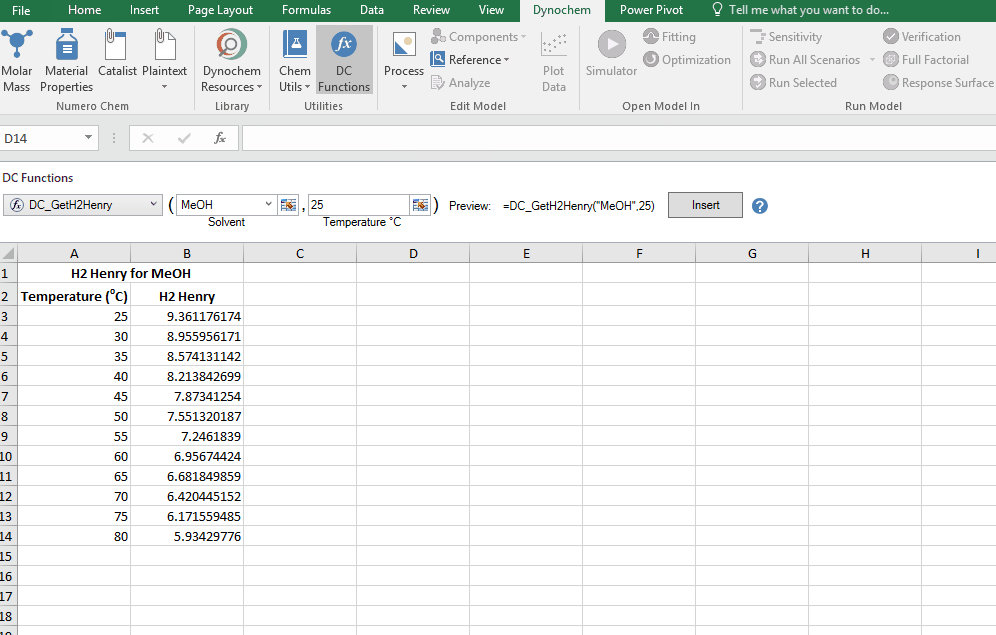
DC_CalcMW
The DC_CalcMW allows you to calculate the molecular weight for a compound from a valid chemical formula.
Syntax
• DC_CalcMW("chemical formula")
Example:
Calculate the molar mass for the following compounds:
i. CH4
ii. Cl2C:
iii. Na[+]
iv. C2O4[2-]
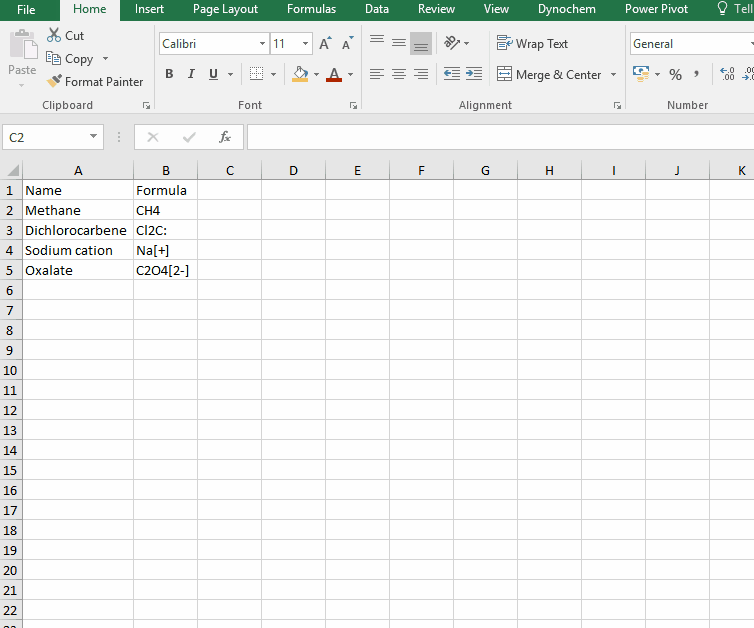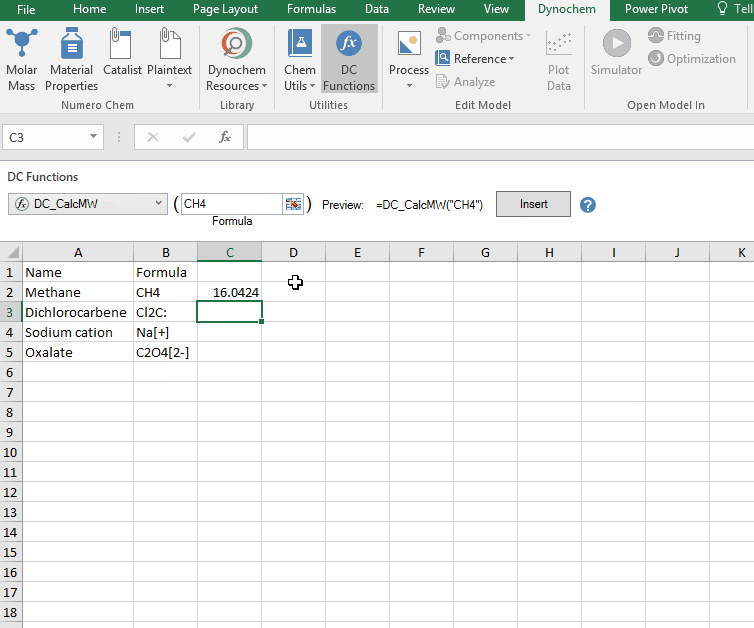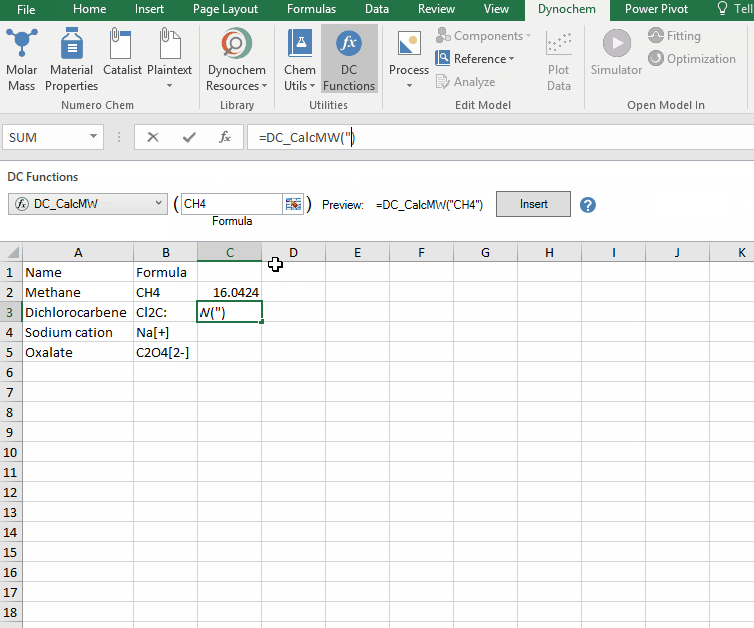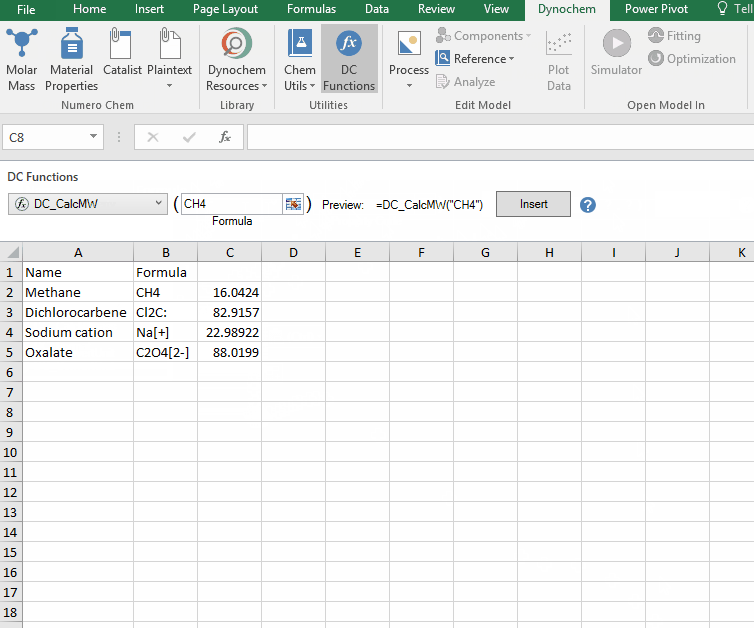
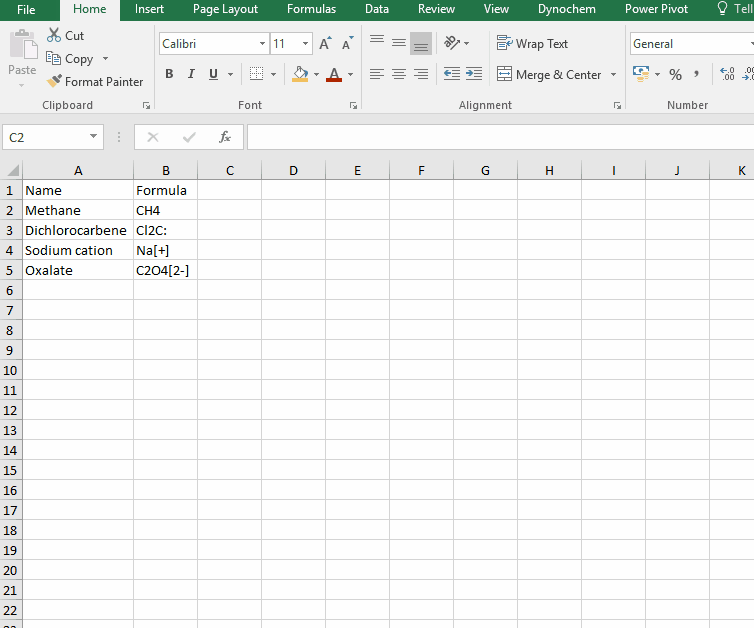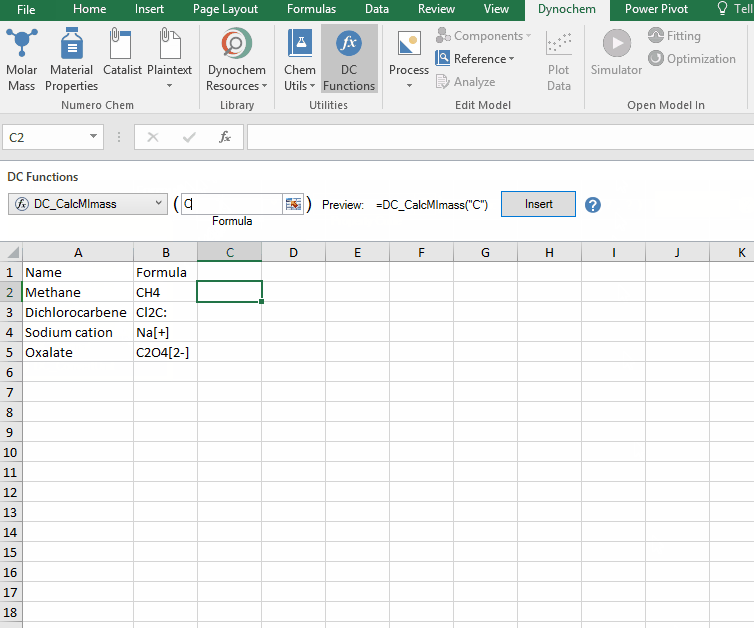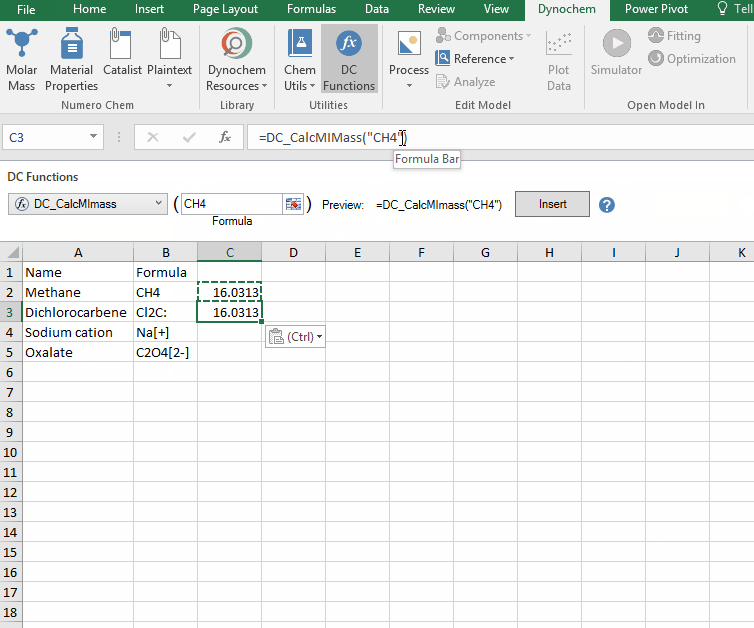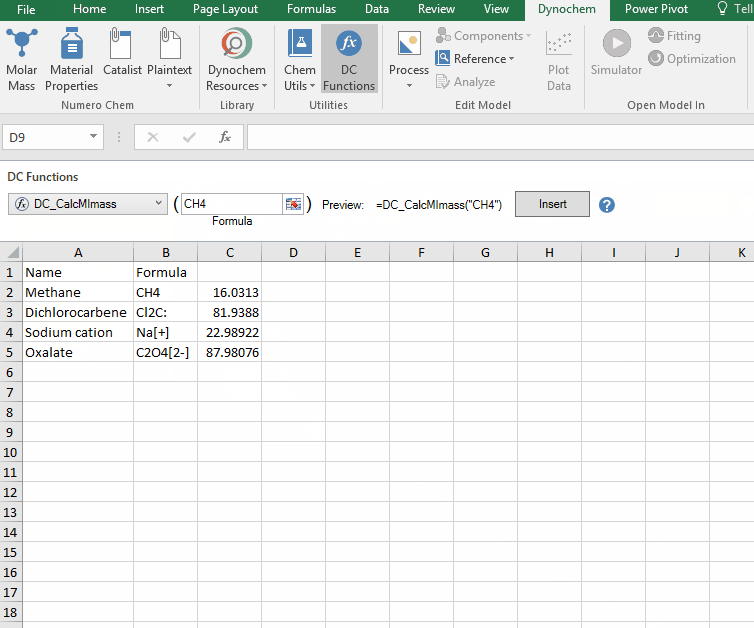
DC_CalcMIMass
The DC_CalcMIMass allows you to calculate the monoisotopic mass for a compound from a valid chemical formula.
Syntax
• DC_CalcMIMass("chemical formula")
Example:
Calculate the monoisotopic mass for the following compounds:
i. CH4
ii. Cl2C:
iii. Na[+]
iv. C2O4[2-]
DC_Format
The DC_Format function allows you to format the chemical formula where all the appropriate numbers are subscripted.
Syntax
• DC_Format(“chemical formula”)
For example:
The following examples show how various compounds can be formatted. For examples, if charged compounds are formatted, the charge is placed inside square bracket
DC_Visvalingam()
This function allows you to reduce your dataset using the Visvalingam-Whyatt algorithm.
Syntax
• DC_Visvalingam("Series", "Points per Series" , "Method")
| Argument |
Description |
| Series |
Select a range of values to reduce |
| Points per Series |
Enter a number. The data series will be reduced to this number of points. |
| Method |
Options: Lead or Individual. Lead will determine the retained points using a single series; Individual will choose retained points separately for each series. |
DC_Regression()
Fits the parameters of a proposed model with predictors to response data using OLS or LASSO method
Syntax
• DC_Regression(Method, Factors, Responses, Features)
| Argument |
Description |
| Method |
Options: OLS or LASSO. Alternatively you can type in "LASSO:MaxIt=1000; Nfold=4; Nlam=25" |
| Factors |
These are the independent/explanatory variables |
| Responses |
This is the response/dependent variable in your regression model |
| Features |
This is a list containing the intercept coefficient, the predictors (x1, x2,...), transformations (e.g. log(x1), exp(x2)), polynomials (x2, x3), and/or interaction terms (x1.x2) |
Output
| b0 |
This is the fitted intercept coefficient
|
|
b1, b2,…
|
These are the fitted slope coefficients
|
|
RMSE
|
Root mean square error. This is an estimate of the standard deviation of the error which is the average amount that the response deviates from the true regression line.
|
|
R2
|
This is the coefficient of determination and is the proportion of variation explained by the model.
|
|
Adjusted R2
|
The is the R2 value adjusted for the number of predictors in the model
|
|
Model DoF
|
This is model degrees of freedom |
|
Error DoF
|
This is the error degrees of freedom |
|
F-statistic
|
This is the value of the F-statistic. When this is larger than the critical F-value, the model is statistically significant |
|
Prob>F
|
This is the p-value for the F-test. When this value is less than the chosen significance level, the model is statistically significant.
|
|
SE
|
This is the standard error for the slope and intercept coefficients
|
|
t-statistic
|
The test-statistic is used to determine whether the intercept or slope coefficients are statistically significant
|
|
Prob>|t| param b0
|
This is the p-value. When this value is less than the significance level (α), then the parameters are statistically significant
|
DC_Prediction
The DC_Prediction function inputs explanatory values (your x values such as temperature, component amounts) into a regression model and returns the predicted values.
Syntax
• DC_Prediction(Factors, Model Definition, Features)
| Argument |
Description |
| Factors |
This is a range that contains your explanatory/independent variables |
| Model definition |
This is a range containing values of the intercept and slope coefficients |
| Features |
This is a list containing the intercept coefficient and explanatory variables all separated by a semi-colon, e.g. y = b0; x1; x2 |
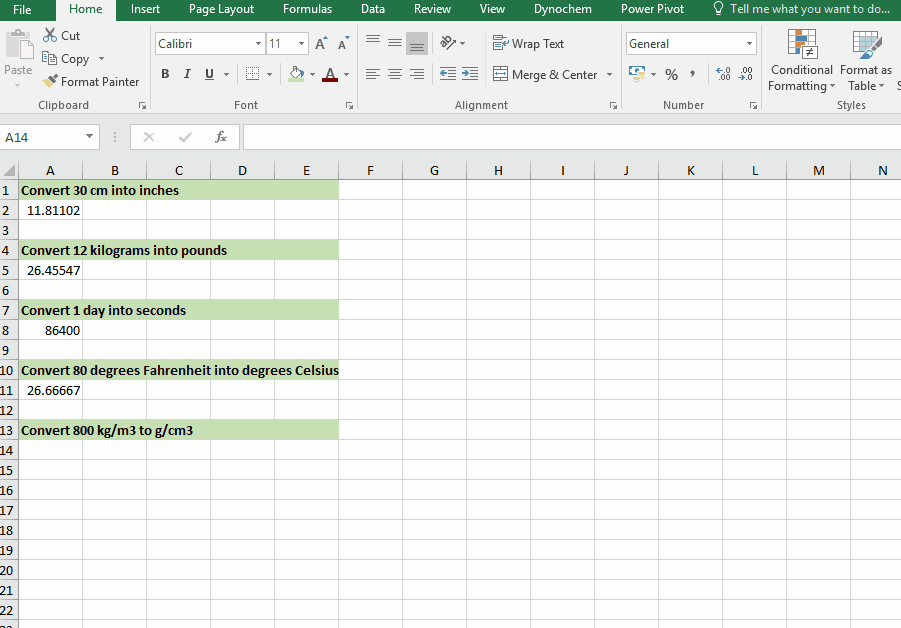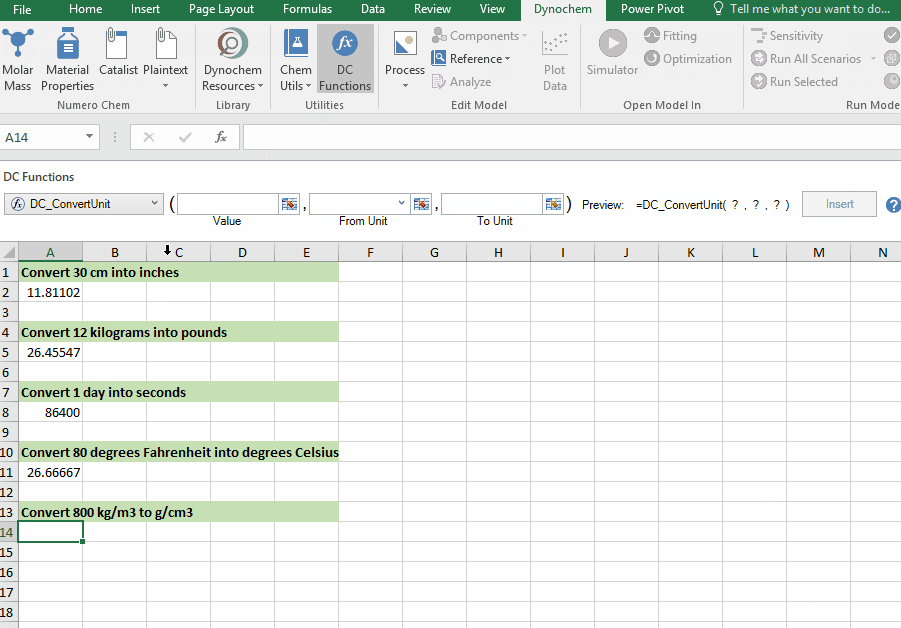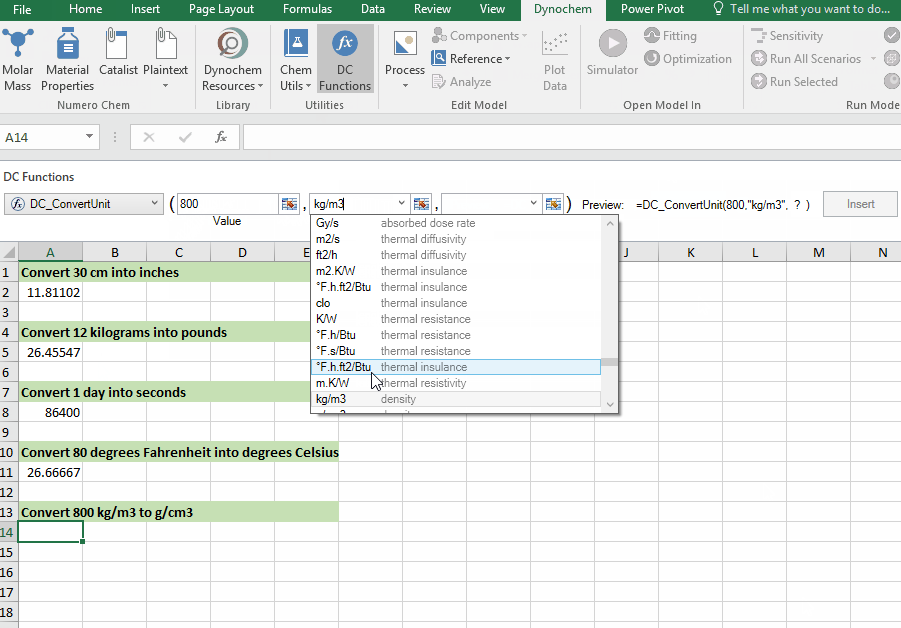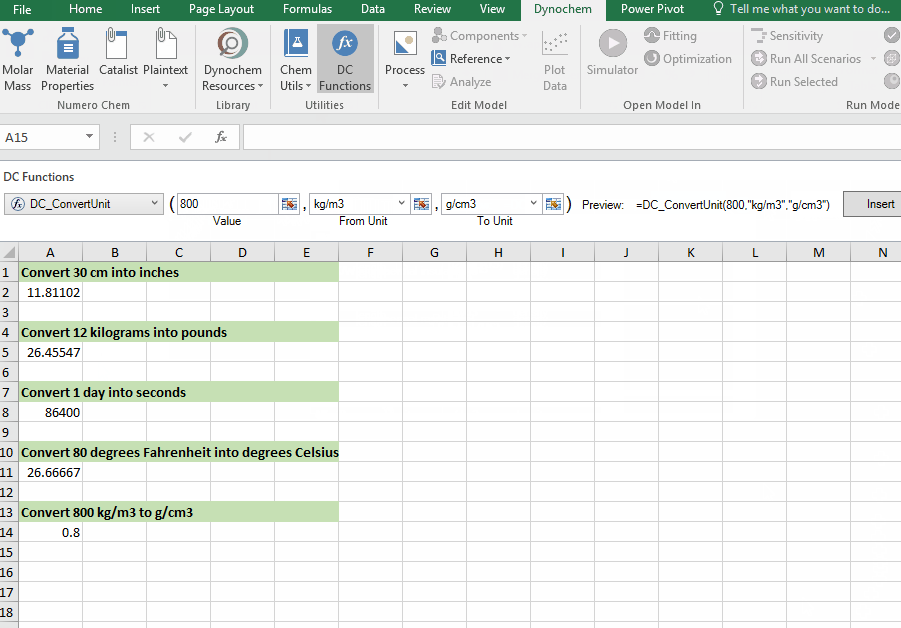
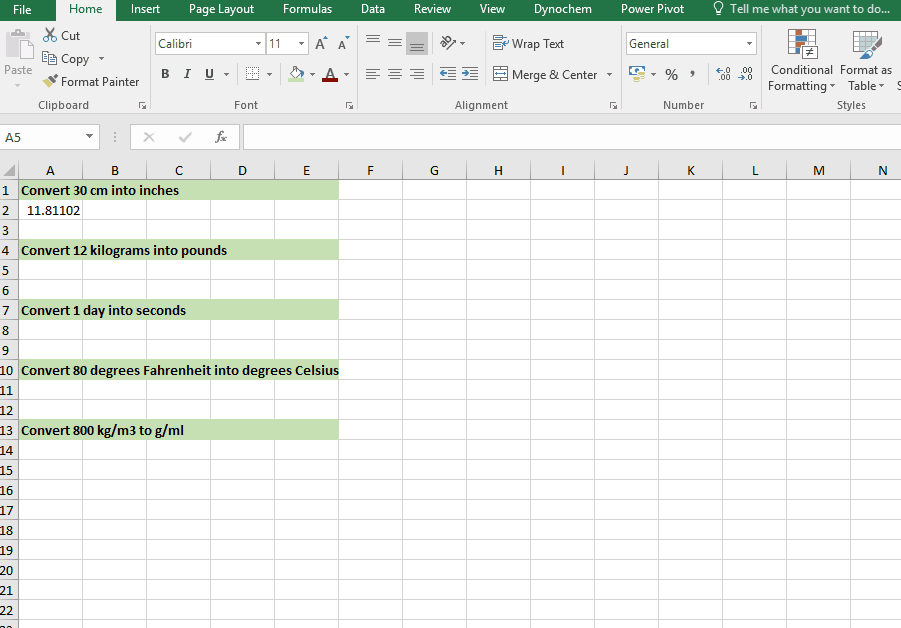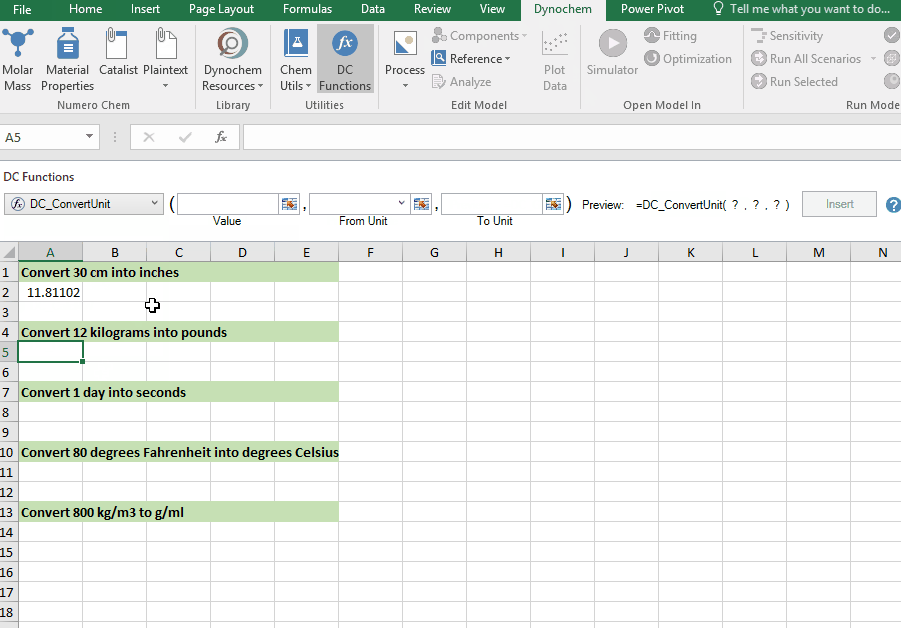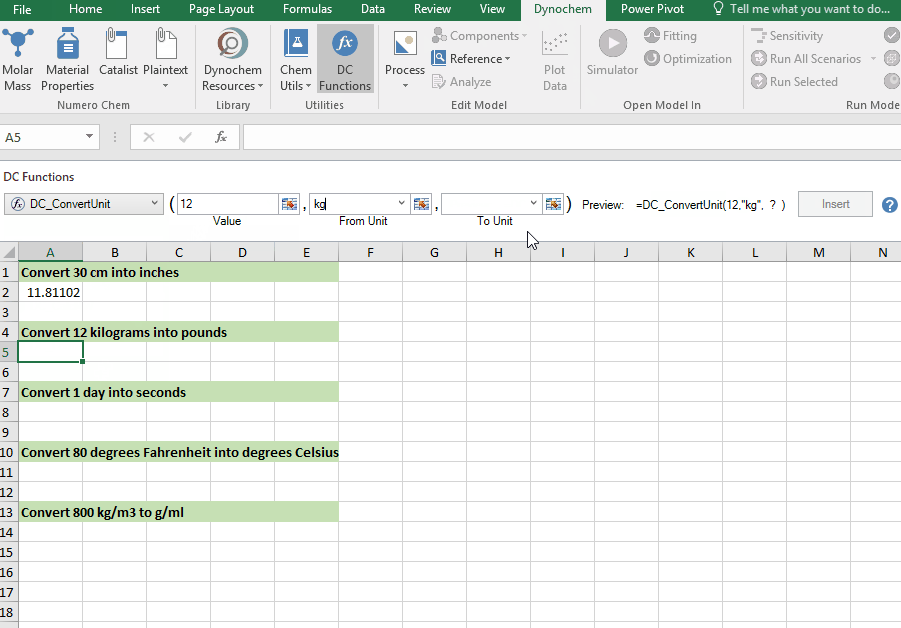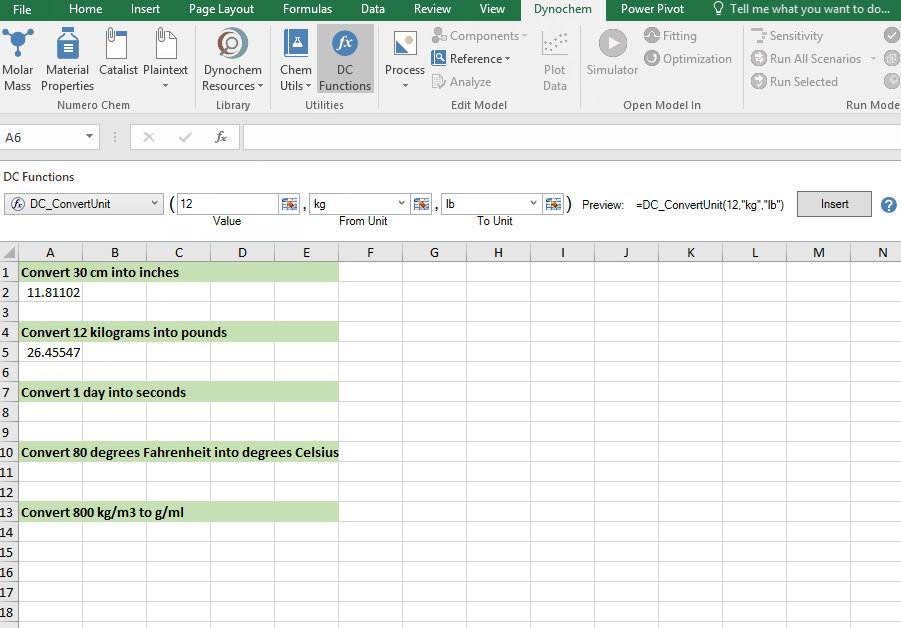
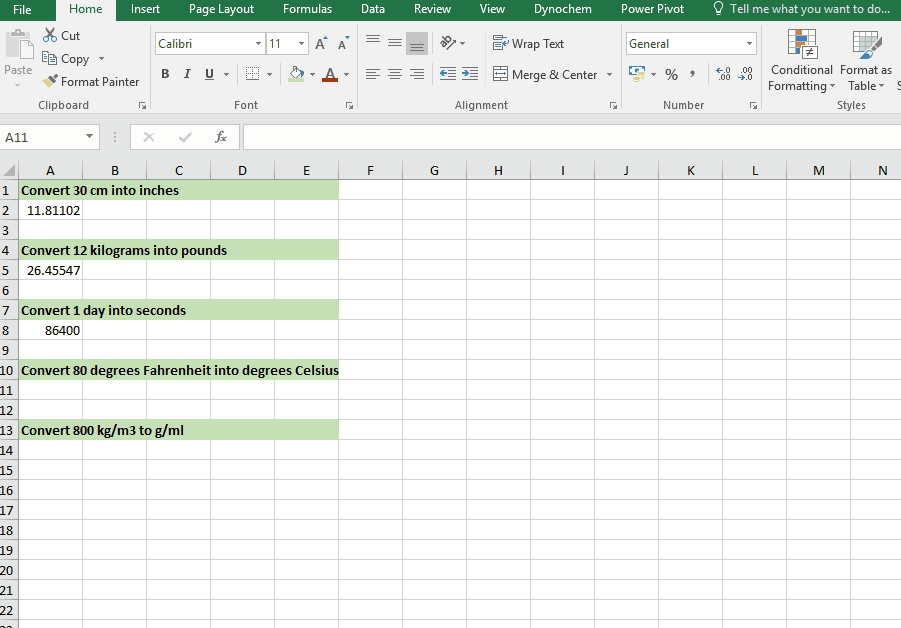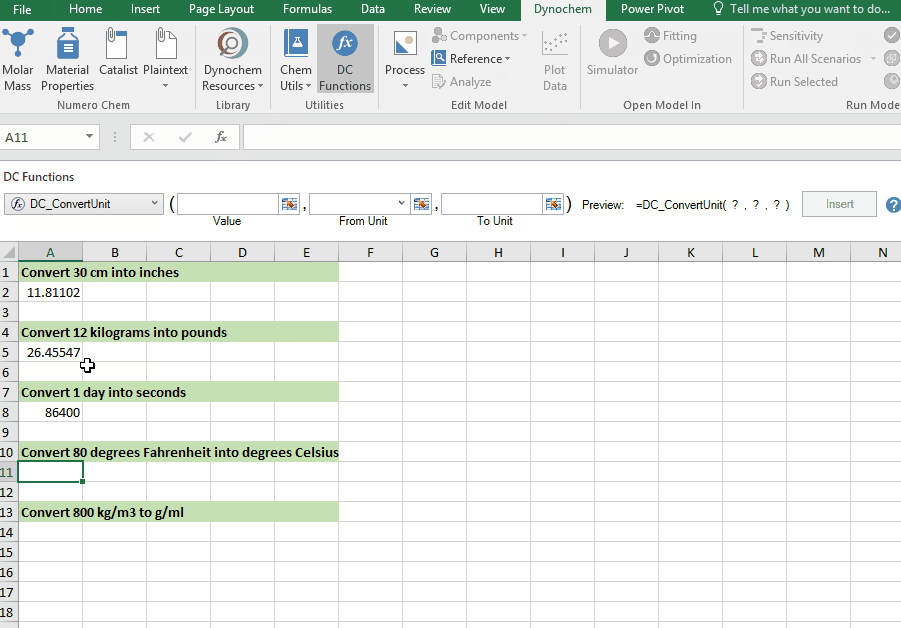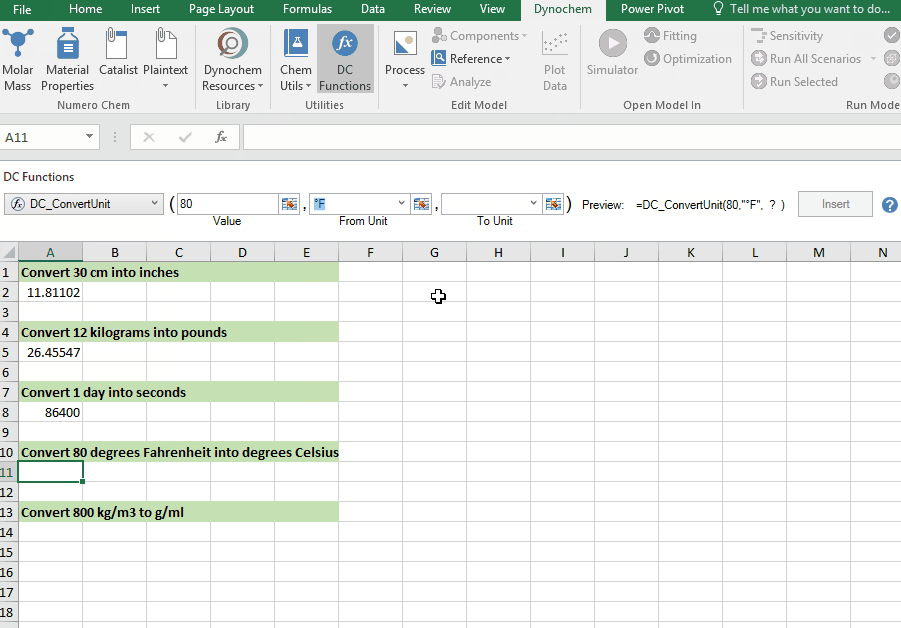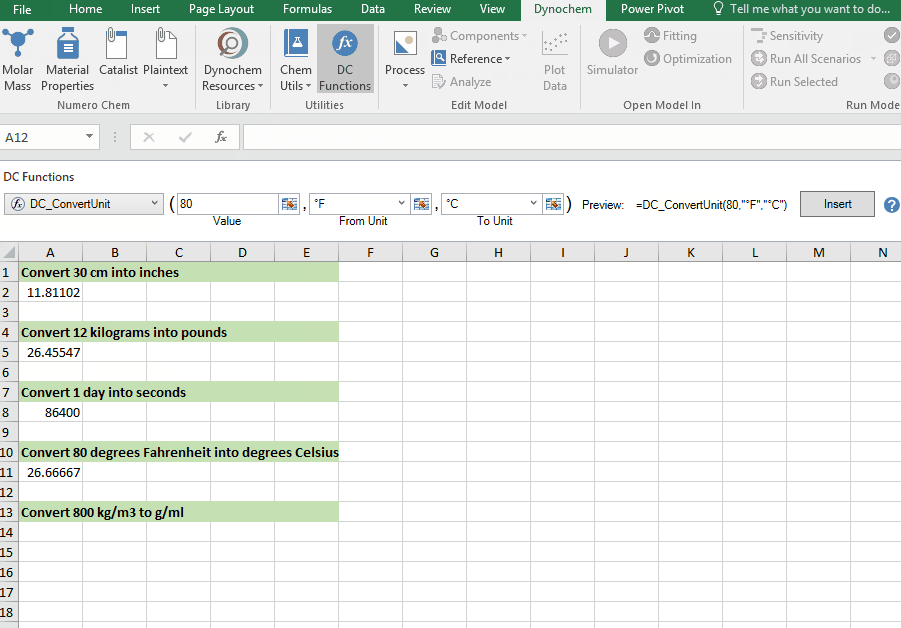
DC_ConvertUnit
The DC_ConvertUnit function allows you to convert values between different units, for example it can convert values in grams to pounds or centimeters to inches.
Syntax
• DC_ConvertUnit(number, "units in quotes", "units in quotes")
Examples:
Convert 30 cm into inches
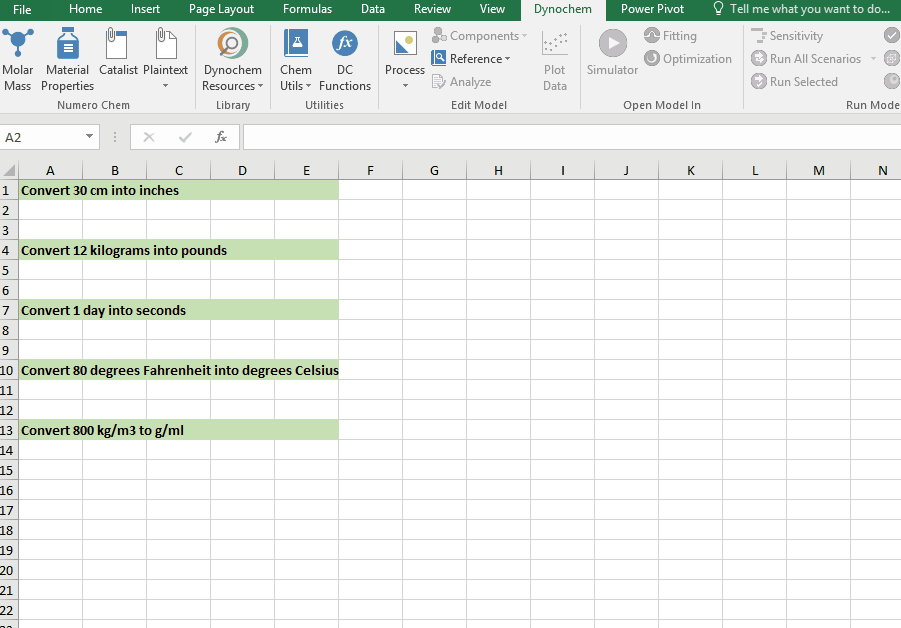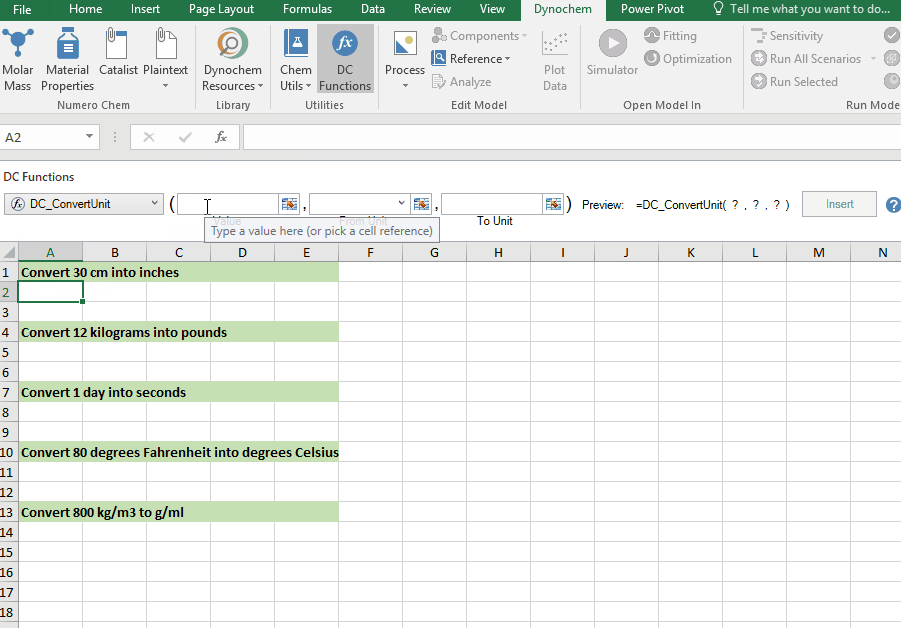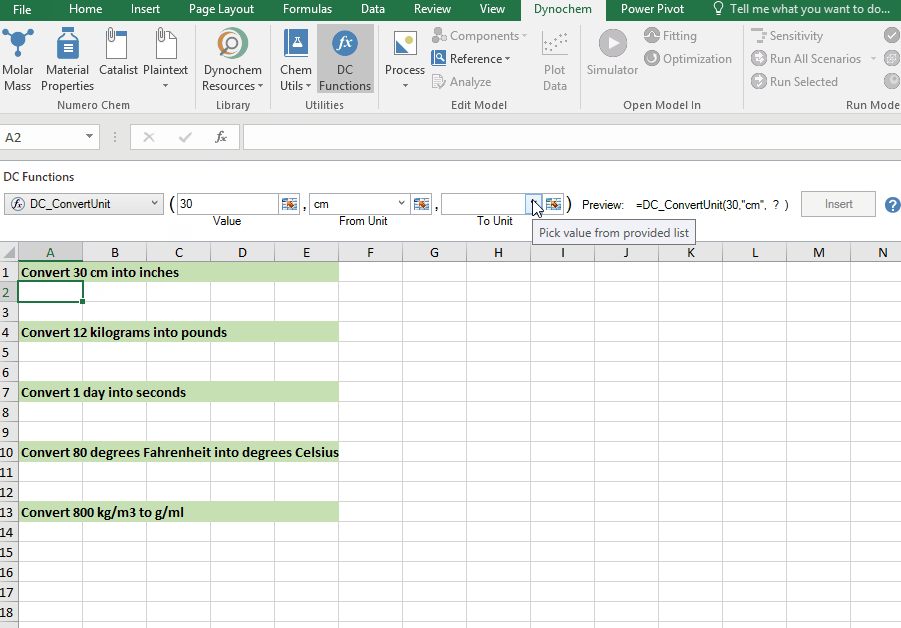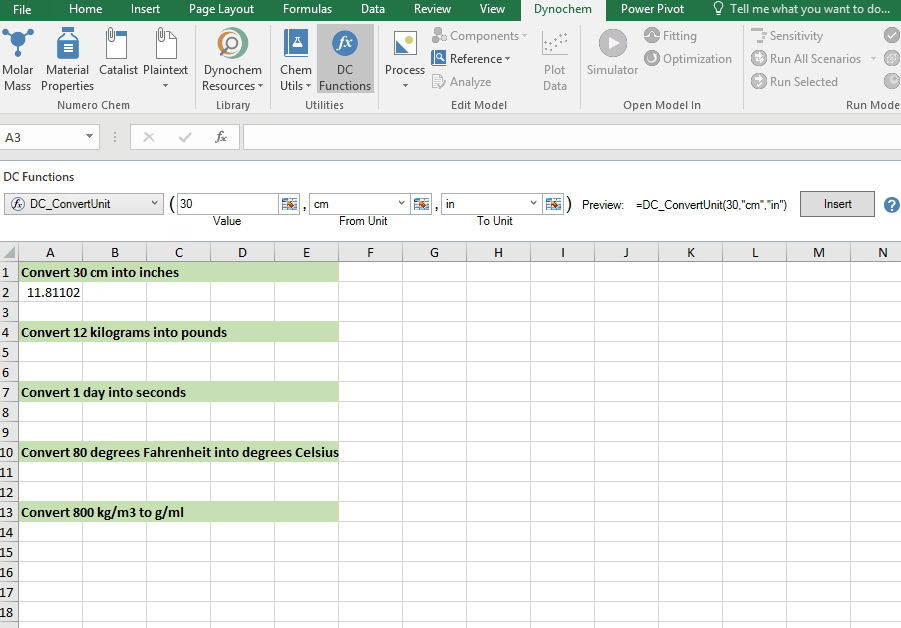
Convert 12 kg into pounds
Convert 1 day into seconds
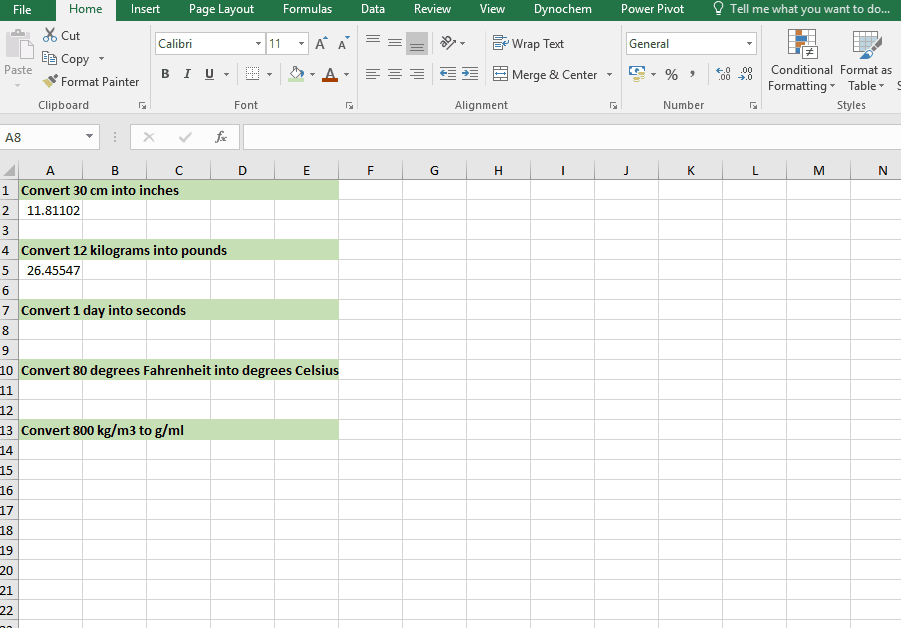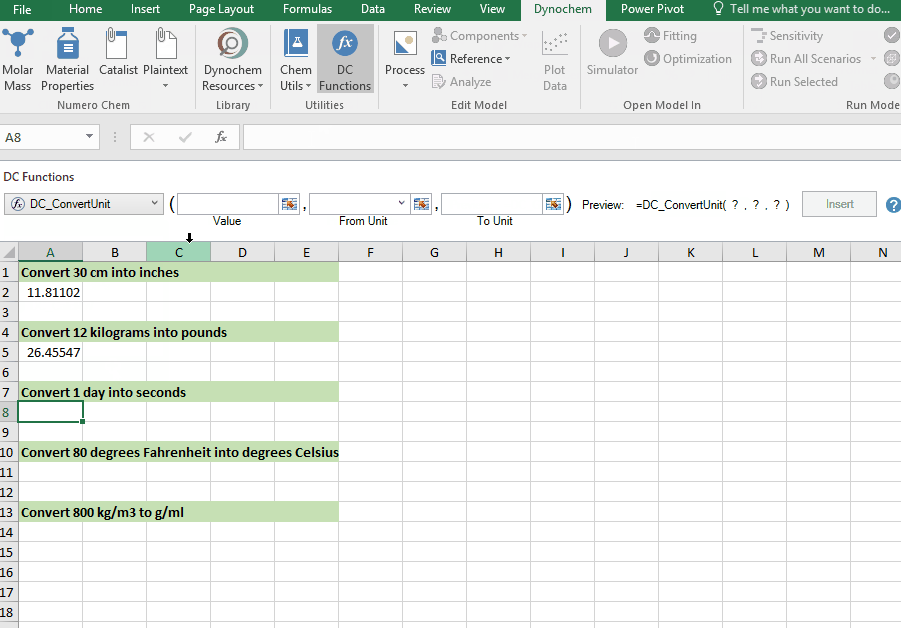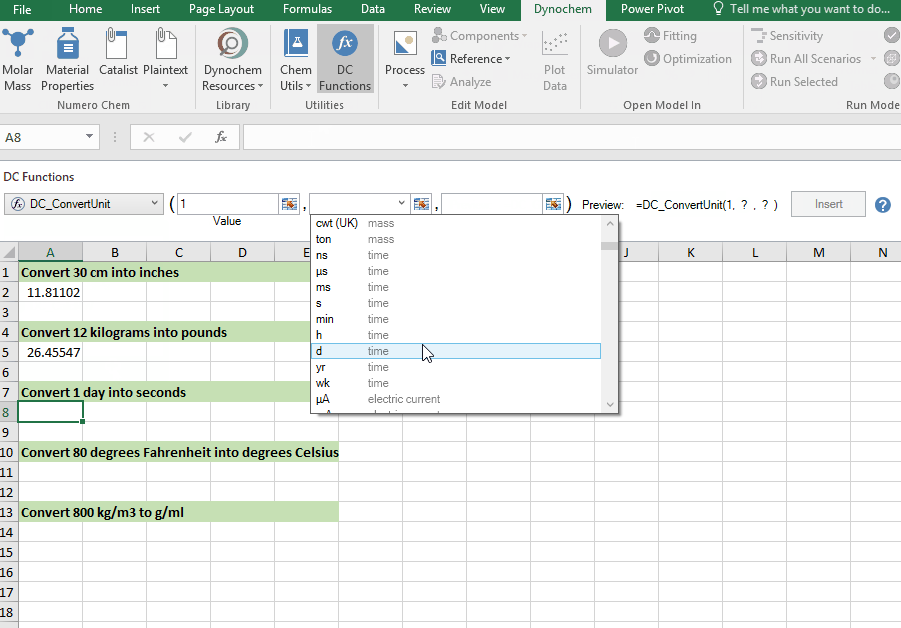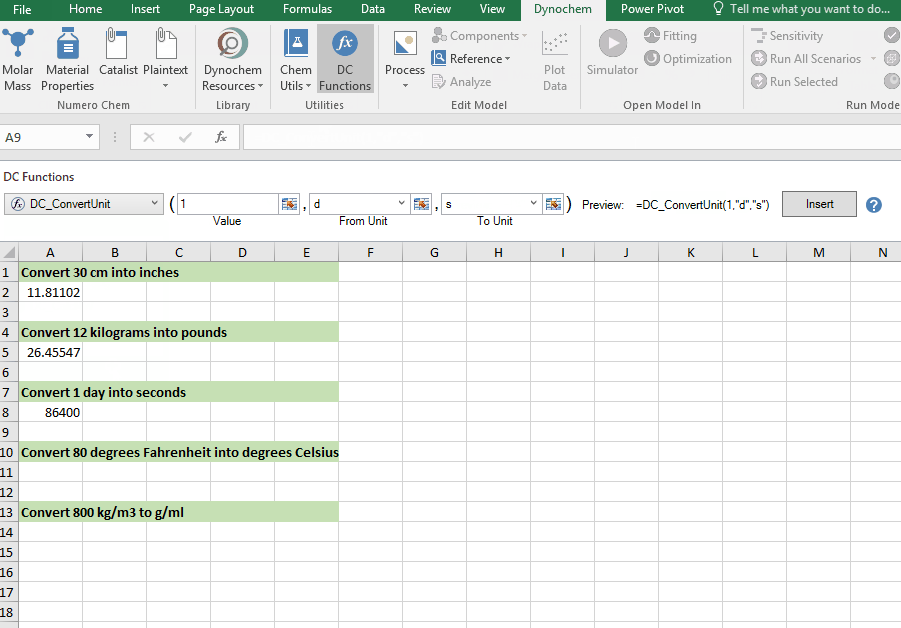
Convert 80 degrees Fahrenheit into degrees Celsius
Convert 998 kg/m3 into g/cm3