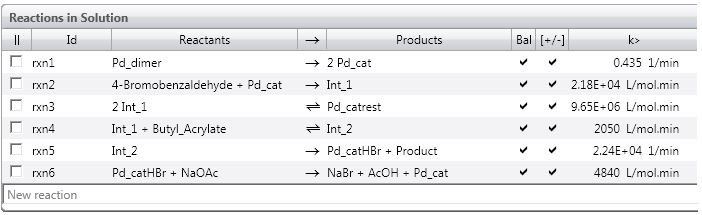
The model will have a Reactions in section for each phase where reactions occur. By default, there is a Reactions in Solution section. For a biphasic system, you might have a Reactions in Organic and a Reactions in Aqueous section. The phases are named and defined in the Process Scheme section, and the names of the Reactions in sections come from the phase names, which can be edited by the user.
You will see below that it is easy to add reaction lines in this section. Please adopt the following best practices in order to develop successful and chemically realistic models:
- Ensure individual reaction lines are balanced in terms of both mass and charge; icons appear on screen to indicate when a reaction is not balanced.
- Because reactions in nature most commonly occur when two molecules meet, ensure that maximum two reactants appear to the left of the reaction arrow; when this is not possible, break your reaction line into two or more lines, with intermediate products. This best practice will lead to reactions that are first or second order unless you override the reaction orders as an advanced option.
- The only exception that we recommend is for catalytic systems, where in some cases it is valid to have the catalyst present as a third component on the left of the reaction arrow. In these cases, remember also to include the catalyst on the right hand side of the reaction arrow so that it is not consumed as the reaction proceeds.
- When your reaction scheme contains many lines, the number of parameters to fit may become large. Consider whether your data are likely to contain enough information to reliably estimate/ fit all of these parameters:
- Reaction lines in your scheme may be set as 'fixed' if you believe that you know their kinetic parameters and do not wish to fit them to data; this is common for fast reactions. Examples include equilibrium reactions, such as those involving dissociation and pH changes, or catalyst adsorption steps.
- Reaction lines may also be set as 'dependent' if you want their kinetics to be linked with those of another reaction line, for example if you have identical reactive sites on multiple species involved in a reaction.
- Both of these measures help capture your chemical knowledge in the model and reduce the number of parameters that need to be fitted to your data. To activate either of these options, click the '=' sign that appears when you hover your mouse pointer over the reaction name and right-click.
- See the reaction schemes shown below for some examples of using these best practices.
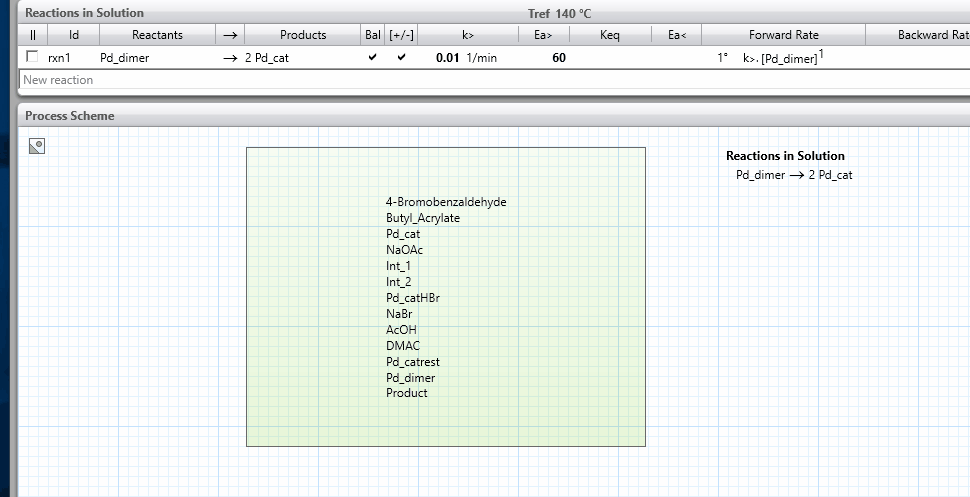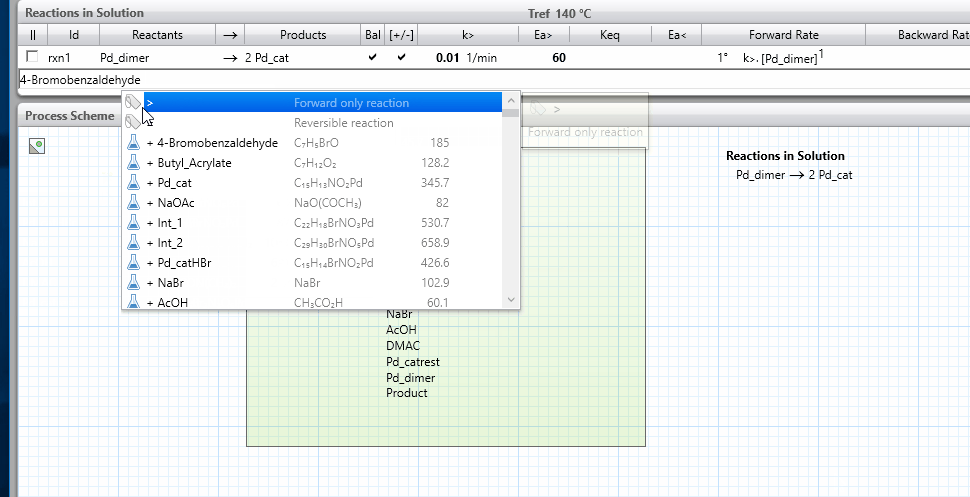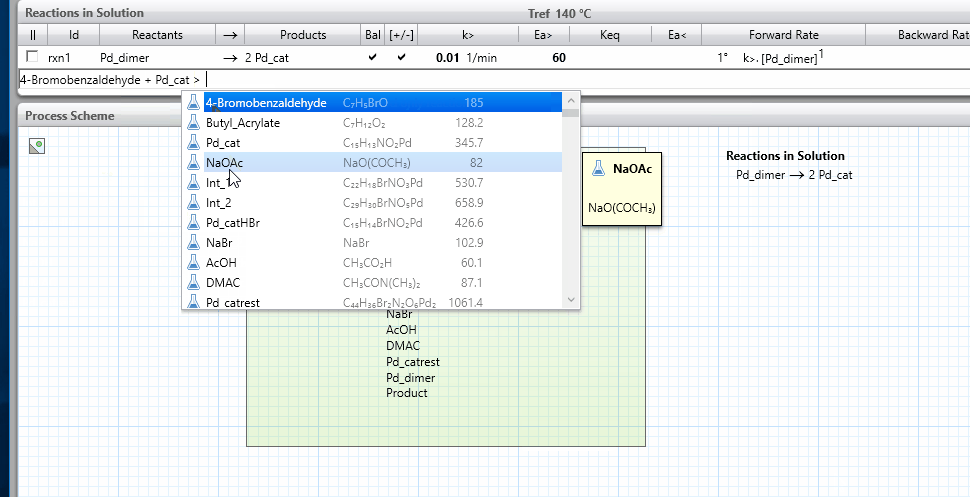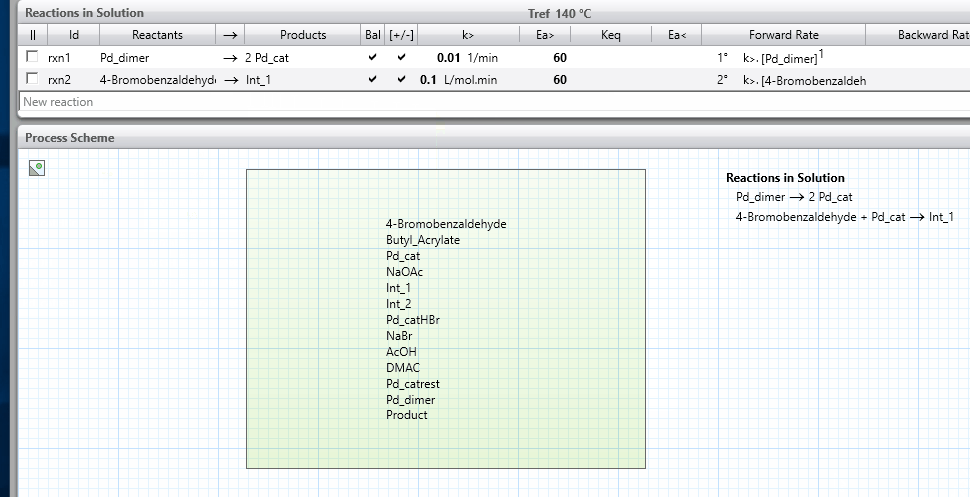
 icon on the right-hand side and this will show the kinetics parameters.
icon on the right-hand side and this will show the kinetics parameters.