What are Material Properties?
Scale-up Suite contains a Materials System with properties information on a wide range of substances related to organic synthesis and bioprocessing. Where possible, we include common synonyms and friendly names for materials, so that our system is easy to use. The principal sources of our material properties are listed at the end of this page. We have curated these properties carefully over two decades.
The Properties item in the Dynochem Excel ribbon provides quick and easy interactive access to this materials information. Similar data may be retrieved into Excel formulae using our DC_functions, also available from the ribbon. The materials system can also be queried from external programs through an API by writing custom code; contact support@scale-up.com for more information and to discuss licensing if you wish to do this.
What Properties are available?
Materials with greatest coverage in our system are solvents. Less data are generally available for reagents, bases and heat transfer fluids. Where possible and the data are available, our properties are temperature-dependent. The full list of properties for pure components is:
Absolute Entropy of Ideal Gas
Acentric Factor
ACS Greenness Env (Air)
ACS Greenness Env (Waste)
ACS Greenness Env (Water)
ACS Greenness Health
ACS Greenness Safety
Antoine A B C unit
Auto Ignition Temperature
CAS RN®
Critical Compressibility Factor
Critical Pressure
Critical Temperature
Critical Volume
Dielectric Constant
Dipole Moment
Electric conductivity
Electric conductivity Temperature
Enthalpy of Formation for Ideal Gas
Enthalpy of Fusion at Melting point
Flash Point
Formatted Hill
Formatted Linear
Formulation for Excipient
Gibbs Energy of Formation for Ideal Gas
GSK Guide 2009 Environmental impact
GSK Guide 2009 Flammability & Explosion
GSK Guide 2009 Health
GSK Guide 2009 Life Cycle Score
GSK Guide 2009 Reactivity/Stability
GSK Guide 2009 Solvent Class
GSK Guide 2009 Waste
Hansen dispersion forces
Hansen hydrogen bonds
Hansen polar forces
Heat of Sublimation
Heat of Vaporization at 25°C
Henry's law constant for H2 at 25°C
Hildebrand parameter
Hill Formula
ICH Residual Solvent Class
Ideal Gas Specific Heat Capacity (Cp) at 25°C
InChI
InChI Key
Linear Formula
Liquid Density at 25°C
Liquid Molar Volume
Liquid Specific Heat Capacity (Cp) at 25°C
Liquid Thermal Conductivity at 25°C
Liquid Viscosity at 25°C
Long Name
Lower Flammability Limit %
Lower Flammability Limit Temperature
Melting Point at 1 atm
Molar Mass
Monoisotopic Mass
Net Standard Enthalpy of Combustion
Normal Boiling Point
Parachor
Radius of Gyration
Refractive Index
Relative Van der Waals Surface Area, Q
Relative Van der Waals Volume, R
Relaxation time constant
Second Virial Coefficient at 25°C
Short Name
SMILES
Solid Density at 25°C
Solid Specific Heat Capacity (Cp) at 25°C
Solubility Parameter
Solvent Family
Solvent Type including ACS Class
Standard State Absolute Entropy
Standard State Enthalpy of Formation
Standard State Gibbs Energy of Formation
Surface Tension at 25°C
Synonym
Triple Point Pressure
Triple Point Temperature
UNIFAC LLE
UNIFAC Modified
UNIFAC VLE
Unique Ingredient Identifier
Upper Flammability Limit %
Upper Flammability Limit Temperature
Van der Waals Reduced Area
Van der Waals Reduced Volume
Vapor Pressure of Liquid at 25°C
Vapor Pressure of Solid at 25°C
Vapor Thermal Conductivity at 25°C
Vapor Viscosity at 25°C
For mixtures, the following parameters and calculation methods are available in DC_functions or utilities, which leverage pure component information from the materials system:
| VLE BIPs |
Group contribution |
Solubility |
| NRTL |
UNIFAC VLE |
R-UNIFAC (solutes) |
| UNIQUAC |
UNIFAC Modified |
Solute activity |
| Solvent activity |
UNIFAC LLE |
Van't Hoff |
|
|
4-parameter models (T,x) |
|
|
Hydrogen, Oxygen, Nitrogen, Carbon Dioxide |
Note that access to material properties for your own proprietary components can be streamlined for users through our Materials Data Service system.
How can I use it?
For basic everyday usage:
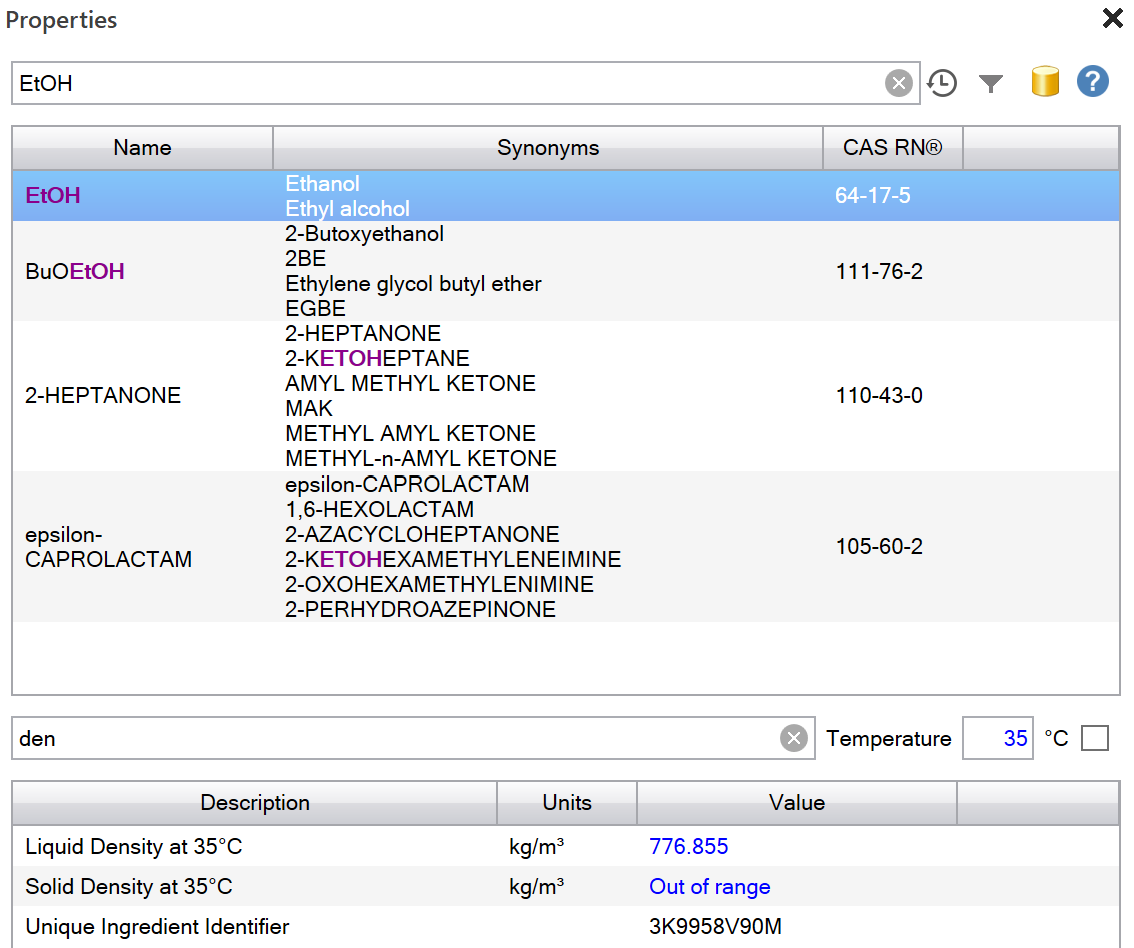
- Open the Properties task pane using the Properties item in the ribbon
- Type the first few letters of the component name of interest, e.g. "EtOH"
- Review the list of properties available in the lower frame
- Optionally type a few letters of the name of a property to filter the results, e.g. "den" for density
- Optionally enter a temperature at which the properties should be displayed
- Optionally write values back to the current worksheet (at the cursor position) by clicking on either Write with Details or Write Value buttons
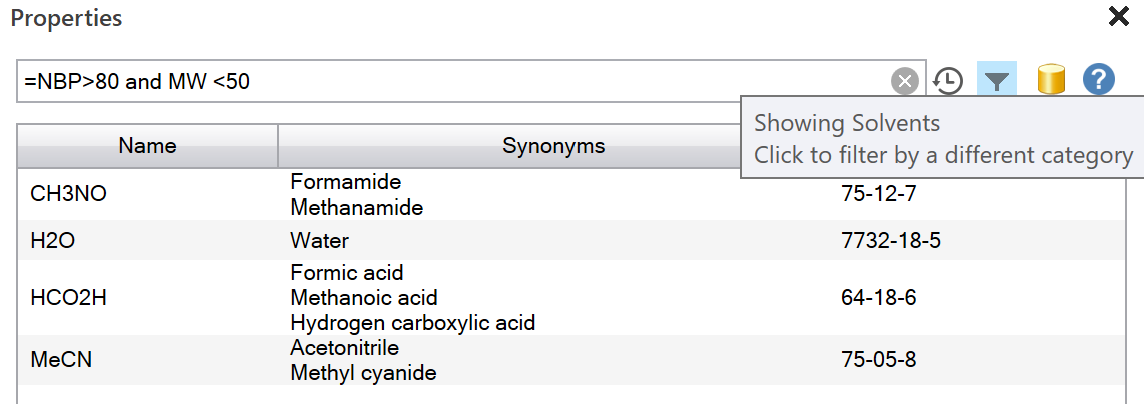
For advanced usage, construct queries using a simple query language:
- Open the Properties task pane
- Start typing a query using the equals sign "="
- Auto-prompts will appear, helping you to complete the query and explaining the meanings of each available property code
- Add further queries using auto-prompts and click Finish when done
- Optionally use the Filter button on the right to select which material categories are shown
- Optionally change the source of the Properties information using the cylinder button (note that changing the source will change the list of properties available)
Sources
We source many pure component physical properties that are temperature-dependent (e.g. liquid density, liquid heat capacity) and also a range of temperature-independent properties from the peer-reviewed DIPPR Database. We source phase equilibrium parameters from the Dortmund Data Bank (DDB) and where gaps exist in their data, we estimate parameters using the modified UNIFAC model. We collate a range of other properties of interest to the user community from various source including ACS and check values against measured or published data where possible. We source excipients information from the FDA website.
For further details about the ACS solvent selection guide, visit the following ACS link. The guide assigns a score from 1 to 10 for each solvent under the respective categories, with a score of 10 being of most concern and a score of 1 suggesting few issues. For the ACS scores, 1=good and 10=bad.
For further details about the GSK solvent selection guide, visit the following link. Each solvent is scored from 1 (red) to 10 (green) to give a relative ranking for every solvent in the guide in each category, where the score is based on data or a physical observable property. Supplementary information is available here. In the GSK scores, 1=bad and 10=good.

